Πρόκειται για επιστημονική εργασία που δημοσιεύτηκε το 2015
(Apidologie, DOI 10.1007/S13592-015-04057)και αφορά εκτεταμένους
πειραματισμούς σε μελίσσια στην Αργεντινή.
Σύμφωνα με την εργασία αυτή, το οξαλικό οξύ εφόσον εμποτιστεί
σε γλυκερίνη και το διάλυμα εμβαπτιστεί σε λωρίδες κυτταρίνης(χαρτόνια),παραμένει
δραστικό για μεγάλο χρονικό διάστημα και με αυτόν τον τρόπο είναι δυνατή μια μόνο
εφαρμογή, χωρίς επαναλήψεις με δράση εναντίον της βαρροα ακόμα και 42 ημέρες
μετά.
Περισσότερα περιοδικό ΜΕΛΙΣΣΟΚΟΜΙΚΗ ΕΠΙΘΕΩΡΗΣΗ ΤΕΥΧΟς
252,ΜΑΡΤΙΟΣ-ΑΠΡΙΛΙΟΣ 2017 ΣΕΛ.101
http://scientificbeekeeping.com/scibeeimages/2016-Beyond-Taktic-pdf.pdf
http://scientificbeekeeping.com/oxalic-shop-towel-updates/
This page is for the sharing information on the extended-release method for oxalic acid application–by dissolving it in glycerin, and then applying to the hive on a cellulose matrix. You can view my article on the subject here: Beyond Taktic. A big thanks to EPA, ARS, and CDPR for working with me toward registering this application method for the benefit of the bee industry!
Warning: this method of treatment is only approved in some countries, but not yet registered in others, including the U.S. It is illegal to apply oxalic acid to a hive in this manner unless it is registered for use in your jurisdiction. However, you may be able to obtain an “Experimental Use Permit” or a “Pesticide Research Authorization” from your State Lead Agency for pesticide regulation.
I do not encourage nor condone the illegal application of any pesticide, including mite treatments to bee hives. The information on this page is solely to report on my progress (working in conjunction with ARS) towards getting this application method approved by EPA , and is not intended to promote illegal use in any way. I get sent anecdotal reports from all over the world, and I assume that anyone reporting such information to me has obtained the proper permit. I neither approve of, nor encourage, applications not sanctioned by local authorities.
Additionally, this application method is a work in progress, with only scant supporting data on efficacy at reducing mite populations. I will be performing formal trials this season.
Update 29 June 2017
Please folks, read all updates before you write me!
I no longer press the towels; instead, I’m mixing in water in order to get the right amount of glycerin into the towels. The formulation that I’m currently testing is:
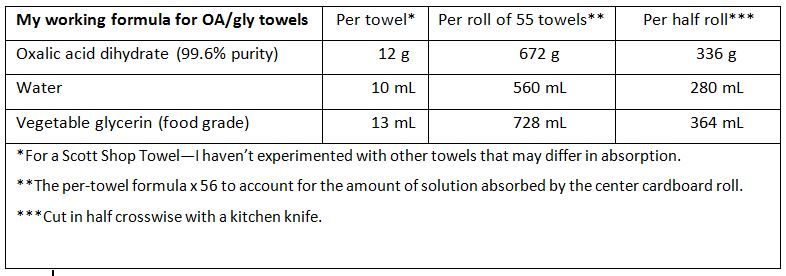
We just finished our midpoint grading of the Calif early-season trial. A heat wave quashed our honey flow, so the colonies did not build up and remove the towels very well in some of the test hives. That said, at 3 weeks into the trial, mite suppression was erratic. I haven’t yet worked the data, but it appears that the towel application method may not be adequate for mite control early in the season. I’ll update soon.
The July issue of ABJ has an extensive update of what I’ve learned to date, with details on preparation methods, and will soon be posted to this website–I’ll update this page when it’s up.
Please realize that this application method is a work in progress–it is not yet approved, and may not result in adequate mite control. I will keep you updated…
Update 8 June 2017
The OA/gly shop towel is currently work in progress, with no claims of efficacy. This update page is informational only; the towels should only be used by those who have received authorization for experimentation.
I’ve sent an extended progress report to ABJ for publication, and will post it here shortly after it’s published. The report covers what I’ve learned to date, and details how I am currently preparing towels (using the 5 May formula) at home.
We’ also now started the first formal trial for EPA registration of the method (in conjunction with ARS) here in Grass Valley, California, with a replicate trial in Georgia (under conditions of higher humidity) to soon begin.

Day Zero of Calif trial. We graded 64 hives for strength, and are here weighing each hive.

We also took honey samples from hives in the Test and Control groups to determine whether treatment substantially increases the natural oxalic acid content of honey.
As a result of testing this spring, I questioned whether a single-towel treatment provides adequate mite control when colonies are building up in spring and early summer–so I was planning on testing three different doses (1,2, or 3 towels per hive). Unfortunately, in two test hives, I found that I can’t increase the dosage by stacking towels one above the other, since this prevents the bees from chewing them adequately. Thus, I changed the protocol shortly before starting the trial, and we are applying only a single dose of three half towels (a 1.5-towel equivalent) per hive (I will also test back-to-back applications).

Applying three half towels to a hive. I’m hoping that the additional exposed edges will increase the rate of distribution of OA in the hive by promoting more chewing at the edges. This application also allows for better vertical movement of bees through the cluster.
Another thing that we’ve learned is that the bees must have access to both the top and bottom of the towels to stimulate them to chew up the towels (and thus distribute the OA)–they do not work well if simply placed under the hive cover. The best position appears to be between the two brood chambers.
Update 5 May 2017
I placed the towels from the previous update into strong colonies, in order to see how quickly the bees would start chewing them–they quickly started chewing holes. So here’s my current best recipe, per Scott Shop Towel:
12 g oxalic acid dihydrate, stirred into
10 mL boiling water until fully dissolved (reheat if necessary), then stir in
13 mL food-grade glycerin.
The above towels, if allowed to air dry, with be a little stiff. Once in the hive, they will quickly rehydrate, and the bees will get to work at removing them (which allows for the mechanical transfer of the solution to the bees’ bodies). The question now is what is the proper dose per hive. Although a dose of one towel per hive in September gave good results last season, the nucs into which I had applied a half towel this spring exhibited unacceptable mite counts when I checked a few days ago. I plan to soon start some formal testing, using different rates of application (including 2 towels laid one on top of the other). Please keep in mind that every one of these updates are merely reports of my preliminary experimentation–NOT recommendations. I still need to confirm efficacy under different conditions, as well as lack of adverse effects upon the colony. There is still much for us to learn!
Addition of an irritant: as I’ve stated before, bees avoid cellulose that is saturated with glycerin. Thus, in order to encourage the bees to chew out the shop towels, one might add an irritant to the glycerin that initiates a hygienic removal response. I’ve thought of using cadaverine or putrescine (and have purchased the raw materials to concoct them), but first was curious about using thymol, since in playing with thymol dissolved in glycerin two years ago, I found that bees readily removed cotton saturated in a thymol/glycerin solution.
Beekeeper Chuck Cook volunteered to run some tests (legal, since he wasn’t using for mite control). He mixed thymol into glycerin at the rates of 0, 0.25, 0.5, 1.0, 1.5 and 2.0 g of thymol per mL of glycerin, and then soaked strips of shop towels in the mixture and presented them to 5 hives. After 14 days, the bees exhibited differences in hygienic removal of the strips:

The above photo is from the hive showing the clearest results–pure glycerin at the bottom, 2 g/mL at the top. It appears that the optimal concentration for eliciting removal would be in the 1 g/mL range. However, Chuck found that two queens were lost during this test–there was simply too much thymol applied in the tests per hive! If one were to add 12 g of thymol to a shop towel, it would also likely be too much. Again, we have much to learn!
Update 2 May 2017
I just came across a very exciting recent finding, by Argentine researcher Matías Maggi, who wondered whether repeated exposure to OA would lead to OA-resistant mites (for some reason the full paper has been taken down, but you can read the abstract here: The Paper). Maggi found that even after being exposed to 64 consecutive treatments by OA dribble over a period of 8 years, the mite population in that apiary was still highly susceptible to OA (surprisingly, apparently even more susceptible than the control group of mites that had never been exposed to OA). This finding suggests that OA, if used judiciously, and in rotation with other treatments with different modes of action, may remain effective for the long term.
Update 30 April 2017
We’re finally getting caught up with beekeeping, so I’m back to kitchen chemistry.
I tried mixing up different proportions of OA, glycerin, and water, for one towel at a time, in order to check the wicking action, crystallization of OA, and texture of the towels. I mixed up individual solutions, each containing 12 g of OA, and differing amounts of glycerin and water. I also ran one wicking test for the highest water concentration (foreground, photo below). I laid out the individual towels over Saran wrap and allowed them to air dry outdoors.

The towels had essentially the same texture once the the water had evaporated off, and whether I used 13 or 14 mL of glycerin. I did notice a slight amount of crystallization of OA on the surfaces after drying. So I placed them in my queen incubator overnight. At broodnest temperature and humidity (95F and 60-70% RH), 12 g of OA in 13 mL of glycerin remains in solution (covered to avoid absorption of water). With the towels, their texture completely changes.

At the elevated temperature and humidity, the glycerin absorbs water, and the towels go from feeling crisp and dry with a slight “oiliness” to feeling very wet and oily. This wetness is what I’m aiming for, since I want the acidic liquid to cling to the bees’ cuticle, but not so oily that the bees avoid chewing the towels.
Update 27 April, 2017
Q: If you use enough ingredients to properly saturate a half roll of shop towels, then apply one shop sheet per hive, if you have, say 5 hives and can’t store the balance, isn’t that a waste of resources?
A: Of course it would be. In the first place, I’m not recommending that anyone use this method–I am merely posting my progress on getting this application method legally registered. I have not yet run formal trials to collect data on efficacy, adverse effects, or contamination of honey stores.
However, for those who can legally experiment with it in their jurisdictions, it is an easy matter to use simple math to make smaller batches. My current working recipe, per full towel, is:
12 g OA dihydrate
13 mL food-grade vegetable glycerin
5 mL distilled water 0r 10 mL
However, I’m finding too much crystallization over time with the above formulation, and need to either increase the glycerin or decrease the amount of OA slightly. And I’m going to experiment with increasing the amount of water.
Update 26 April 2017
Q: Have you been able to successfully store the towels and or solution? I can foresee smaller operations wanting to get in on this and not necessarily need 55 towels at one go. Would you recommend just preparing less solution or can it be stored after it’s made.
A: I’ve been storing a few rolls of prepared towels at cool room temperature for a couple of months. As prepared, the towels get a bit more “delicate.” Coupled with some crystallization of the OA, it becomes more difficult to unroll the towels without tearing them (I may need to either increase the glycerin or decrease the OA). In addition, over time, some of the OA may react with the glycerin to form esters (which may or may not have effects on the bees or degree of efficacy for mite control). So I suggest that towels be made fresh for use, and not stored for prolonged periods of time.
Update 21 April 2017
It’s been too crazy with bee work to run experiments, but I’m getting feedback from other beekeepers. One compared February treatment with Apivar vs. OA/gly shop towels (2 half towels/hive), prior to blueberry pollination in North Carolina, and recorded frame strength, frames of brood, and starting and ending alcohol wash counts. After 46 days, there were no differences in frame strength, frames of brood, or queen loss between the groups. Mite counts started close to zero in both groups, and declined in both groups. Unfortunately, he didn’t run an untreated control group, so we can’t calculate efficacy.
After the 46 days, the colonies had removed most of the towels, which is my goal:

Update 6 April 2017
In response to the two reports below that OA/gly treatments had killed bees, I ran a quick and dirty experiment. I made up 10 medium-strength 5-frame nucs with 2nd-yr queens, and laid a half an OA/gly shop towel across the top bars of each one. Weather conditions were initially cool and rainy.
I checked the nucs each day for any signs of dead bees at the entrance, and never saw any. After a week, the bees had not yet chewed the towels to any extent, so I added another 5-frame box of drawn comb above, in order to give the bees access to the tops of the towels. At that time, due to the cool, wet weather, I fed each nuc a half gallon of 1:1 sugar syrup via a top feeder.
I still saw no dead bees at the entrances, and then the weather warmed, and the bees took the syrup, and then began foraging on a nice nectar and pollen flow. Yesterday I went into 6 of the nucs to check for signs of adverse effects. Every nuc that I checked was thriving and expanding as well as other untreated nucs in my operation. The brood patterns were lovely:

A typical brood pattern of a treated nuc. Note the partially-chewed towel that I lifted with my hive tool in order to remove the frame. I do not understand why some others are reporting adverse effects.
Of interest, in one of the nucs that I checked, the syrup had dripped onto the towel, giving the bees an opportunity to consume oxalic/glycerin/sugar syrup solution. Again, even in this nuc I observed no signs of adverse effects. In this particular experiment, I was only interested in seeing whether there would be adverse effects from treatment of small nucs under poor weather conditions in early spring. Due to the small clusters and poor weather, I did not take mite washes, so cannot comment on efficacy of the treatment. I plan to begin a larger, controlled trial on nucs next week.
Update 12 March 2017–updated measurements into grams. Until we better understand the chemistry, I suggest heating the mixture quickly in order to dissolve the OA, and then pouring it immediately onto the towels and allow it to cool. The point is to avoid holding the OA/gly solution at high temp any longer than necessary.
Update 11 March 2017
A beekeeper just reported to me that he placed 2-3 cardboard strips following the Argentine formula into 15 nucs and killed them all. I’ve not yet tested on nucs, so be careful!
Another email today: “Two weeks ago, I treated one strong hive in a remote yard with glycerin and oxalic acid saturated in a Brawny Dine-a-Max towel – I like the untreated Dine-a-Max towel as a trap for small hive beetles, and thought it might do well as a carrier for the gly/ox. When I checked the hive last week, there was a large pile of dead bees on the ground at the entrance of the hive – probably the nurse bees, since the dying field bees probably flew away before dying.
Folks, experimentation carries risk. I’ll be testing this application method thoroughly this season, and will report my results.
Update 10 March 2017
I’m currently working with EPA and Dr. Jay Evans at USDA ARS to add this method as an approved application method (so that it can be used legally). The current sticking point is to get it approved for use while honey supers are on, which will apparently require us to do further testing, and to have EPA set an acceptable maximum residue level for OA in honey. Luckily, natural honeys and especially some honeydews contain a fair amount of OA, as do many veggies. So this likely won’t be an insurmountable problem, but will need to work through the bureaucratic requirements. A big thanks to EPA, ARS, and CDPR for working with me for the benefit of the bee industry!
Good news, my experimentation is now legal! California DPR has issued me a Pesticide Research Authorization to run field experiments with the OA/gly towels (snip below).
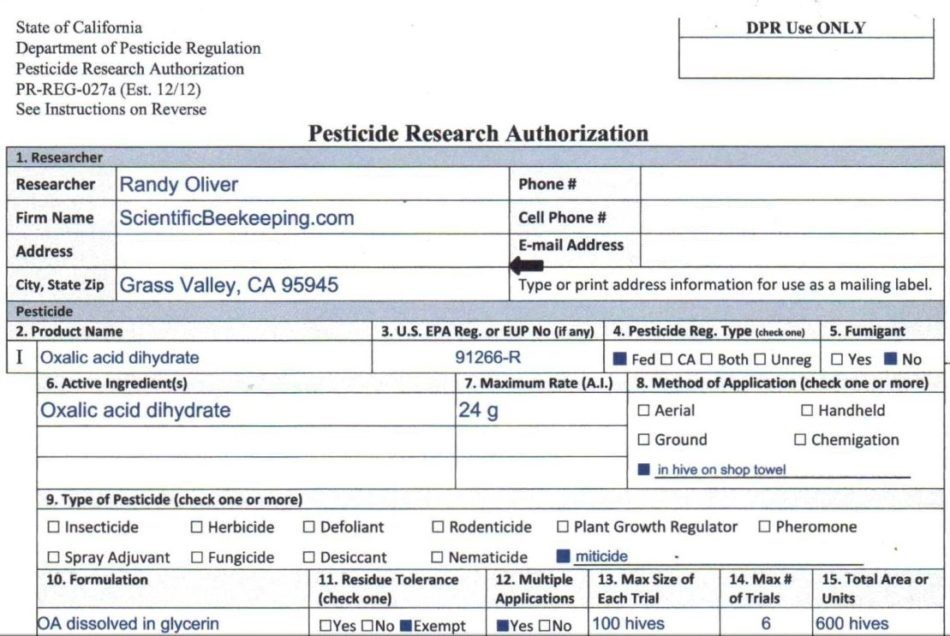
There are quite a few beekeepers worldwide currently experimenting with this method of application–keep in mind that OA/gly extended release is not yet registered for application in the U.S. I encourage others to apply for experimental permits in their jurisdictions.

Photo sent to me by a commercial beekeeper running tests (name withheld for his protection).
I had a brainstorm–instead of using rolled towels, to instead use the folded ones (below) that come in an 85-towel pack, and are dispensed one at a time like Kleenex tissues.

I purchased a stainless steel steam table insert that fit the stack of towels perfectly, and poured in the solution (below).

Result: it worked beautifully! I was sure that this would be the answer for easy towel dispensing. But alas, the proof of the pudding is in the eating. When I attempted to pull the towels out, the OA/gly prevented them from separating easily (below).. It looks as though I’m back to the rolls.

OK, you may have noticed that I’m not wearing protective nitrile gloves. I’ve gotten so casual with these towels, that when testing in the kitchen, I often handle them with bare fingers, and then immediately wash the solution off afterwards. I’ve not noticed any skin irritation, but you sure wouldn’t want to rub your eyes or pick your nose! Best to ALWAYS wear gloves.
Current best formulation from my testing to get the maximum amount of OA into a usable shop towel that is not too “oily” to discourage removal by the bees:
Per towel Per full roll of 55 towels
OA crystals 12 g 672 g
Glycerin 13 ml 728 ml (917 g at room temp)
Water 5 ml 280 ml (280 g at room temp)
Be sure to measure each ingredient before adding it to the mix, in order to avoid the overshooting that can easily occur if you try to weigh the ingredients as you add them to the mixing pot.
For a roll of 55 towels, I multiplied the amounts by 56 (in order to account for the absorption by the cardboard roll in the center). Tip, if you measure the glycerin in a measuring cup, after you pour it out, place the cup in a microwave for a few seconds to heat the remaining glycerin, and the remainder will pour out easily).
We may not need this much OA in a towel. My preliminary testing with cardboard strips to replicate Maggi’s trials in Argentina indicated that it required 4 strips per box for adequate varroa reduction. That worked out to 40 g of OA per box, or 80 g per double-deep hive. A shop towel using the above formula holds only 12 g per hive. Its increased efficacy over the cardboard strips is apparently due to the chewing-removal action by the bees.
Tip: I noticed yesterday that it appeared to help to heat the water first, then add the OA. This appeared to allow me to break up the chunks better than when I add the OA to the hot glycerin first (I need to confirm this).
Some other recent questions:
Q: I don’t remember reading how long you leave this treatment in place.
A: In the first place, this application method is not yet approved for use in the U.S. You may be able to obtain an experimental use permit from your State department of pesticide regulation. That said, the application method is designed for an extended release over a period of roughly one month, by which point the bees should have removed most of the shop towel. Mite reduction is not immediate, and from my limited testing, appears to take about a month. Therefore, if legal in your state or country, apply it as soon as the mite infestation rate (as determined by alcohol wash) exceeds 2 mites/100 bees.
Q: Can it be used with supers in place?
A: According to Maggi’s testing, this treatment does not appear to increase OA residues in honey. I’m asking EPA to approve it for use with honey supers on.
Q: Does the ambient temperature matter?
A: I’ve tested at up to 100°F (at low humidity), and did not observe any adverse effects. I’ve also tested during our California winter (for adverse effects only–not efficacy), during sustained cold rain and some freezing temperatures. Again, I observed no adverse effects upon the bees or brood.
Q: When sourcing your Oxalic acid/Glycerin etc have you looked at different grades of OA? I see you have food grade gly. I have been sifting through OA msds sheets from what we have locally available and the chemical composition varies wildly from agricultural bore cleaner or wood bleach to technical grade OA with prices that vary even more. The lower end “bore cleaner” OA Dihydrate has Sulphate at 1000ppm and some metal elements included, Iron at 100ppm and Lead at 50ppm.
A: The EPA requires registered “Distributors” of OA for varroa treatment to certify the purity of the OA. Currently, Brushy Mountain is the only registered distributor. I’m working with another registrant to bring prepared towels to market at an inexpensive cost.
Update 12 Feb 2017
I checked several hives yesterday that have had a OA/gly towel in them for the past two months during rainy, cold spring buildup. There does not appear to be any adverse effect upon the colony.
Please remember that this formulation is not yet approved for use in the U.S. (I am in the process of obtaining an Experimental Use Permit for the State of California). It is against the law to apply OA to hives in an unapproved manner. I’m working with EPA and ARS to get this application method added to the label, but it’s a slow process.
Meanmwhile, I’ve been doing lots of kitchen chemistry to figure out how to avoid the need to press the excess OA/glycerin out of the shop towels in order to obtain a texture that the bees will remove. It appears the total glycerin should not exceed 13 mL per towel. This amount of glycerin will hold 12 g of OA, without the OA coming out of solution at hive temp.
I think that I’ve finally hit upon a formula that works. It is difficult to get the solution to wick by capillary action into the full length of a roll of shop towels. (For non U.S. readers, the Scott shop towels are a cellulose heavy-duty, highly absorbent towel http://www.kcprofessional.com/products/wipers/general/scott-shop-towels).
So here’s a step-by-step process that appears to make the ideal towel, although I need to confirm for efficacy in the hive.

Step 1. Use a sharp kitchen knife to cut the roll of towels exactly in half crosswise.

Step 2. Find a stainless steel saucepan that just fits the roll (stood on end). The optimal formula appears to be (per towel) 12 g of OA dihydrate, 13 mL of glycerine, and 5 mL of water. Multiplied by 55 towels + the cardboard roll = 56/2, for final amounts per half roll of towels of:
Yes, adding water turned out to be OK, so long as I use slightly more
glycerin in mL than OA in grams. The water will mostly evaporate from
the towels, depending upon temp and humidity. I show a graduated
cylinder above on the right (most accurate), and a measuring cup on the
left.
Weigh out the 336 g of OA (I actually use a more accurate beam balance, but it’s not critical).

You can preheat the water and glycerin in the microwave, or on the stove. It doesn’t appear to be critical when you add the water, but if you add it first to the glycerin before adding the OA, it may help to prevent the formation of oxalic esters during the heating.

Heat to 140-160°F. Higher temps will cause degradation of the OA and accelerate ester formation (both of which we wish to avoid).

Add the OA crystals, and use a stainless steel spoon (it may discolor) to stir while you dissolve the OA. Wear nitrile gloves and safety glasses! Keep the burner on the mixture while stirring, but do not allow the temp to exceed 160F. With a modicum of caution, this is safe to do.

As soon as the mixture is completely clarified, remove it from the heat and place the pan on a plastic tray.

Preheat the roll of dry towels for 1 minute in the microwave–this preheating helps the solution to wick into the roll. Although you can insert a piece of 1-1/4″ PVC pipe into the roll, I find no benefit from doing so, since the cardboard cylinder will come loose anyway. Also no benefit to leaving the wrapper around the roll, since I get better absorption if the roll is allowed to expand.

Carefully place the roll into the hot solution. The pan should first be placed on a plastic tray (not as shown). The solution will quickly wick up part way. When it gets to about 1/3rd of the way up, then flip the roll.

Use kitchen tongs to flip the roll. The mixture will not immediately irritate your skin, and can be easily washed off with warm water. But it is sticky, and will get on all surfaces if you’re not careful. It is really important not to allow traces of the solution to get on surfaces, as it is slowly corrosive, and a hazard. You don’t want it ending up in your eyes! Keep warm water and a wet sponge on hand for wiping surfaces. A little bit of baking soda dissolved in water will immediately neutralize any acid.

The solution will wick up from the other end.

You may find it to be of benefit to flip the roll again. The roll will absorb every last drop of solution. Allow the roll to cool, and for the water to evaporate off (I’m not sure that this is necessary, as it would also occur later in the hive). You can then place the roll into a secure plastic container or ziplock bag for storage.
I found that when I held towels in the solution at 150F for a few days (with or without water), that the acid dissolved the towel. I don’t observe this happening at room temp, but have not had time to test to determine how long the treated towels can be stored before losing strength. The Aluen CAP strips claim to be good for an extended storage period. I will be performing tests to see whether the OA or towels degrade with extended storage. For now, probably best to make them up fresh.
One would then apply two half sheets of towel per hive for a treatment, which would apply 12 g of OA to the hive.
I’m not completely sold on the rolled towels, and have the folded version on order, which I will test when they arrive.
I’m buried in email from those wanting more info. Please only email me if you have something important to offer, or a serious question. If you are not comfortable with accurate weighing, measuring, and safe handling of acids, YOU SHOULD NOT ATTEMPT TO DO THIS!
I am encouraging one cooperating bee supply company to bring an inexpensive premade version to market.
Update 24 Jan 2017
There has been lots of response to my article on OA/gly in ABJ. I’m in communication with EPA to get this application method approved.
I’m in the middle of a trial of OA/gly shop towels on 6 hives during our wet, cold winter. As of today, after ~3 weeks, the colonies are not showing any adverse effects upon the bees.
I’m currently working on is the best ratio of OA to glycerin, and the best amount per towel. The problem is that the towels don’t want to evenly absorb the optimum amount of glycerin to leave them less “oily” so that the bees will chew at the towels. I’m trying to figure out how to get a full roll of towels to absorb the right amount of solution without the need for squeezing out the excess.
I tried 700g OA in 700ml of glycerin, heated to 160F, and the roll of towels preheated 1.5 minutes in the microwave. I poured the hot solution into a tall, narrow asparagus pot, and set one end of the towel roll into the pot. When the solution had soaked half way up, I flipped the roll over, and placed the roll into a warm oven. After 15 minutes, the solution has still not absorbed to the center of the roll. So I left it for another hour in the warm oven. This proved to be a mistake, since the acid degraded the lower part of the roll into a soft mess.
The 700ml of glycerin also appeared to be too much–leaving the towels too “oily” feeling.
I’m now going to experiment with adding isopropyl alcohol (boiling point 180F) to the solution, and decreasing the amount of glycerin.
http://scientificbeekeeping.com/scibeeimages/2016-Beyond-Taktic-pdf.pdf
http://scientificbeekeeping.com/oxalic-shop-towel-updates/
Oxalic shop towel updates
This page is for the sharing information on the extended-release method for oxalic acid application–by dissolving it in glycerin, and then applying to the hive on a cellulose matrix. You can view my article on the subject here: Beyond Taktic. A big thanks to EPA, ARS, and CDPR for working with me toward registering this application method for the benefit of the bee industry!
Warning: this method of treatment is only approved in some countries, but not yet registered in others, including the U.S. It is illegal to apply oxalic acid to a hive in this manner unless it is registered for use in your jurisdiction. However, you may be able to obtain an “Experimental Use Permit” or a “Pesticide Research Authorization” from your State Lead Agency for pesticide regulation.
I do not encourage nor condone the illegal application of any pesticide, including mite treatments to bee hives. The information on this page is solely to report on my progress (working in conjunction with ARS) towards getting this application method approved by EPA , and is not intended to promote illegal use in any way. I get sent anecdotal reports from all over the world, and I assume that anyone reporting such information to me has obtained the proper permit. I neither approve of, nor encourage, applications not sanctioned by local authorities.
Additionally, this application method is a work in progress, with only scant supporting data on efficacy at reducing mite populations. I will be performing formal trials this season.
I’m overloaded with emails regarding this subject. PLEASE do not write me unless you have read the entire article, plus all the updates below. Thank you for your consideration.
If you have previously viewed this page, please reload it to ensure that your computer is not showing you an outdated cached copy. Only then, if after reading all the updates, you have questions or you’ve figured out ways to improve the method, please write me and I’ll post here.Update 29 June 2017
Please folks, read all updates before you write me!
I no longer press the towels; instead, I’m mixing in water in order to get the right amount of glycerin into the towels. The formulation that I’m currently testing is:

We just finished our midpoint grading of the Calif early-season trial. A heat wave quashed our honey flow, so the colonies did not build up and remove the towels very well in some of the test hives. That said, at 3 weeks into the trial, mite suppression was erratic. I haven’t yet worked the data, but it appears that the towel application method may not be adequate for mite control early in the season. I’ll update soon.
The July issue of ABJ has an extensive update of what I’ve learned to date, with details on preparation methods, and will soon be posted to this website–I’ll update this page when it’s up.
Please realize that this application method is a work in progress–it is not yet approved, and may not result in adequate mite control. I will keep you updated…
Update 8 June 2017
The OA/gly shop towel is currently work in progress, with no claims of efficacy. This update page is informational only; the towels should only be used by those who have received authorization for experimentation.
I’ve sent an extended progress report to ABJ for publication, and will post it here shortly after it’s published. The report covers what I’ve learned to date, and details how I am currently preparing towels (using the 5 May formula) at home.
We’ also now started the first formal trial for EPA registration of the method (in conjunction with ARS) here in Grass Valley, California, with a replicate trial in Georgia (under conditions of higher humidity) to soon begin.

Day Zero of Calif trial. We graded 64 hives for strength, and are here weighing each hive.

We also took honey samples from hives in the Test and Control groups to determine whether treatment substantially increases the natural oxalic acid content of honey.
As a result of testing this spring, I questioned whether a single-towel treatment provides adequate mite control when colonies are building up in spring and early summer–so I was planning on testing three different doses (1,2, or 3 towels per hive). Unfortunately, in two test hives, I found that I can’t increase the dosage by stacking towels one above the other, since this prevents the bees from chewing them adequately. Thus, I changed the protocol shortly before starting the trial, and we are applying only a single dose of three half towels (a 1.5-towel equivalent) per hive (I will also test back-to-back applications).

Applying three half towels to a hive. I’m hoping that the additional exposed edges will increase the rate of distribution of OA in the hive by promoting more chewing at the edges. This application also allows for better vertical movement of bees through the cluster.
Another thing that we’ve learned is that the bees must have access to both the top and bottom of the towels to stimulate them to chew up the towels (and thus distribute the OA)–they do not work well if simply placed under the hive cover. The best position appears to be between the two brood chambers.
Update 5 May 2017
I placed the towels from the previous update into strong colonies, in order to see how quickly the bees would start chewing them–they quickly started chewing holes. So here’s my current best recipe, per Scott Shop Towel:
12 g oxalic acid dihydrate, stirred into
10 mL boiling water until fully dissolved (reheat if necessary), then stir in
13 mL food-grade glycerin.
The above towels, if allowed to air dry, with be a little stiff. Once in the hive, they will quickly rehydrate, and the bees will get to work at removing them (which allows for the mechanical transfer of the solution to the bees’ bodies). The question now is what is the proper dose per hive. Although a dose of one towel per hive in September gave good results last season, the nucs into which I had applied a half towel this spring exhibited unacceptable mite counts when I checked a few days ago. I plan to soon start some formal testing, using different rates of application (including 2 towels laid one on top of the other). Please keep in mind that every one of these updates are merely reports of my preliminary experimentation–NOT recommendations. I still need to confirm efficacy under different conditions, as well as lack of adverse effects upon the colony. There is still much for us to learn!
Addition of an irritant: as I’ve stated before, bees avoid cellulose that is saturated with glycerin. Thus, in order to encourage the bees to chew out the shop towels, one might add an irritant to the glycerin that initiates a hygienic removal response. I’ve thought of using cadaverine or putrescine (and have purchased the raw materials to concoct them), but first was curious about using thymol, since in playing with thymol dissolved in glycerin two years ago, I found that bees readily removed cotton saturated in a thymol/glycerin solution.
Beekeeper Chuck Cook volunteered to run some tests (legal, since he wasn’t using for mite control). He mixed thymol into glycerin at the rates of 0, 0.25, 0.5, 1.0, 1.5 and 2.0 g of thymol per mL of glycerin, and then soaked strips of shop towels in the mixture and presented them to 5 hives. After 14 days, the bees exhibited differences in hygienic removal of the strips:

The above photo is from the hive showing the clearest results–pure glycerin at the bottom, 2 g/mL at the top. It appears that the optimal concentration for eliciting removal would be in the 1 g/mL range. However, Chuck found that two queens were lost during this test–there was simply too much thymol applied in the tests per hive! If one were to add 12 g of thymol to a shop towel, it would also likely be too much. Again, we have much to learn!
Update 2 May 2017
I just came across a very exciting recent finding, by Argentine researcher Matías Maggi, who wondered whether repeated exposure to OA would lead to OA-resistant mites (for some reason the full paper has been taken down, but you can read the abstract here: The Paper). Maggi found that even after being exposed to 64 consecutive treatments by OA dribble over a period of 8 years, the mite population in that apiary was still highly susceptible to OA (surprisingly, apparently even more susceptible than the control group of mites that had never been exposed to OA). This finding suggests that OA, if used judiciously, and in rotation with other treatments with different modes of action, may remain effective for the long term.
Update 30 April 2017
We’re finally getting caught up with beekeeping, so I’m back to kitchen chemistry.
I tried mixing up different proportions of OA, glycerin, and water, for one towel at a time, in order to check the wicking action, crystallization of OA, and texture of the towels. I mixed up individual solutions, each containing 12 g of OA, and differing amounts of glycerin and water. I also ran one wicking test for the highest water concentration (foreground, photo below). I laid out the individual towels over Saran wrap and allowed them to air dry outdoors.

The towels had essentially the same texture once the the water had evaporated off, and whether I used 13 or 14 mL of glycerin. I did notice a slight amount of crystallization of OA on the surfaces after drying. So I placed them in my queen incubator overnight. At broodnest temperature and humidity (95F and 60-70% RH), 12 g of OA in 13 mL of glycerin remains in solution (covered to avoid absorption of water). With the towels, their texture completely changes.

At the elevated temperature and humidity, the glycerin absorbs water, and the towels go from feeling crisp and dry with a slight “oiliness” to feeling very wet and oily. This wetness is what I’m aiming for, since I want the acidic liquid to cling to the bees’ cuticle, but not so oily that the bees avoid chewing the towels.
Update 27 April, 2017
Q: If you use enough ingredients to properly saturate a half roll of shop towels, then apply one shop sheet per hive, if you have, say 5 hives and can’t store the balance, isn’t that a waste of resources?
A: Of course it would be. In the first place, I’m not recommending that anyone use this method–I am merely posting my progress on getting this application method legally registered. I have not yet run formal trials to collect data on efficacy, adverse effects, or contamination of honey stores.
However, for those who can legally experiment with it in their jurisdictions, it is an easy matter to use simple math to make smaller batches. My current working recipe, per full towel, is:
12 g OA dihydrate
13 mL food-grade vegetable glycerin
5 mL distilled water 0r 10 mL
However, I’m finding too much crystallization over time with the above formulation, and need to either increase the glycerin or decrease the amount of OA slightly. And I’m going to experiment with increasing the amount of water.
Update 26 April 2017
Q: Have you been able to successfully store the towels and or solution? I can foresee smaller operations wanting to get in on this and not necessarily need 55 towels at one go. Would you recommend just preparing less solution or can it be stored after it’s made.
A: I’ve been storing a few rolls of prepared towels at cool room temperature for a couple of months. As prepared, the towels get a bit more “delicate.” Coupled with some crystallization of the OA, it becomes more difficult to unroll the towels without tearing them (I may need to either increase the glycerin or decrease the OA). In addition, over time, some of the OA may react with the glycerin to form esters (which may or may not have effects on the bees or degree of efficacy for mite control). So I suggest that towels be made fresh for use, and not stored for prolonged periods of time.
Update 21 April 2017
It’s been too crazy with bee work to run experiments, but I’m getting feedback from other beekeepers. One compared February treatment with Apivar vs. OA/gly shop towels (2 half towels/hive), prior to blueberry pollination in North Carolina, and recorded frame strength, frames of brood, and starting and ending alcohol wash counts. After 46 days, there were no differences in frame strength, frames of brood, or queen loss between the groups. Mite counts started close to zero in both groups, and declined in both groups. Unfortunately, he didn’t run an untreated control group, so we can’t calculate efficacy.
After the 46 days, the colonies had removed most of the towels, which is my goal:
Update 6 April 2017
In response to the two reports below that OA/gly treatments had killed bees, I ran a quick and dirty experiment. I made up 10 medium-strength 5-frame nucs with 2nd-yr queens, and laid a half an OA/gly shop towel across the top bars of each one. Weather conditions were initially cool and rainy.
I checked the nucs each day for any signs of dead bees at the entrance, and never saw any. After a week, the bees had not yet chewed the towels to any extent, so I added another 5-frame box of drawn comb above, in order to give the bees access to the tops of the towels. At that time, due to the cool, wet weather, I fed each nuc a half gallon of 1:1 sugar syrup via a top feeder.
I still saw no dead bees at the entrances, and then the weather warmed, and the bees took the syrup, and then began foraging on a nice nectar and pollen flow. Yesterday I went into 6 of the nucs to check for signs of adverse effects. Every nuc that I checked was thriving and expanding as well as other untreated nucs in my operation. The brood patterns were lovely:

A typical brood pattern of a treated nuc. Note the partially-chewed towel that I lifted with my hive tool in order to remove the frame. I do not understand why some others are reporting adverse effects.
Of interest, in one of the nucs that I checked, the syrup had dripped onto the towel, giving the bees an opportunity to consume oxalic/glycerin/sugar syrup solution. Again, even in this nuc I observed no signs of adverse effects. In this particular experiment, I was only interested in seeing whether there would be adverse effects from treatment of small nucs under poor weather conditions in early spring. Due to the small clusters and poor weather, I did not take mite washes, so cannot comment on efficacy of the treatment. I plan to begin a larger, controlled trial on nucs next week.
Update 12 March 2017–updated measurements into grams. Until we better understand the chemistry, I suggest heating the mixture quickly in order to dissolve the OA, and then pouring it immediately onto the towels and allow it to cool. The point is to avoid holding the OA/gly solution at high temp any longer than necessary.
Update 11 March 2017
A beekeeper just reported to me that he placed 2-3 cardboard strips following the Argentine formula into 15 nucs and killed them all. I’ve not yet tested on nucs, so be careful!
Another email today: “Two weeks ago, I treated one strong hive in a remote yard with glycerin and oxalic acid saturated in a Brawny Dine-a-Max towel – I like the untreated Dine-a-Max towel as a trap for small hive beetles, and thought it might do well as a carrier for the gly/ox. When I checked the hive last week, there was a large pile of dead bees on the ground at the entrance of the hive – probably the nurse bees, since the dying field bees probably flew away before dying.
Folks, experimentation carries risk. I’ll be testing this application method thoroughly this season, and will report my results.
Update 10 March 2017
I’m currently working with EPA and Dr. Jay Evans at USDA ARS to add this method as an approved application method (so that it can be used legally). The current sticking point is to get it approved for use while honey supers are on, which will apparently require us to do further testing, and to have EPA set an acceptable maximum residue level for OA in honey. Luckily, natural honeys and especially some honeydews contain a fair amount of OA, as do many veggies. So this likely won’t be an insurmountable problem, but will need to work through the bureaucratic requirements. A big thanks to EPA, ARS, and CDPR for working with me for the benefit of the bee industry!
Good news, my experimentation is now legal! California DPR has issued me a Pesticide Research Authorization to run field experiments with the OA/gly towels (snip below).

There are quite a few beekeepers worldwide currently experimenting with this method of application–keep in mind that OA/gly extended release is not yet registered for application in the U.S. I encourage others to apply for experimental permits in their jurisdictions.

Photo sent to me by a commercial beekeeper running tests (name withheld for his protection).
I had a brainstorm–instead of using rolled towels, to instead use the folded ones (below) that come in an 85-towel pack, and are dispensed one at a time like Kleenex tissues.

I purchased a stainless steel steam table insert that fit the stack of towels perfectly, and poured in the solution (below).

Result: it worked beautifully! I was sure that this would be the answer for easy towel dispensing. But alas, the proof of the pudding is in the eating. When I attempted to pull the towels out, the OA/gly prevented them from separating easily (below).. It looks as though I’m back to the rolls.

OK, you may have noticed that I’m not wearing protective nitrile gloves. I’ve gotten so casual with these towels, that when testing in the kitchen, I often handle them with bare fingers, and then immediately wash the solution off afterwards. I’ve not noticed any skin irritation, but you sure wouldn’t want to rub your eyes or pick your nose! Best to ALWAYS wear gloves.
Current best formulation from my testing to get the maximum amount of OA into a usable shop towel that is not too “oily” to discourage removal by the bees:
Per towel Per full roll of 55 towels
OA crystals 12 g 672 g
Glycerin 13 ml 728 ml (917 g at room temp)
Water 5 ml 280 ml (280 g at room temp)
Be sure to measure each ingredient before adding it to the mix, in order to avoid the overshooting that can easily occur if you try to weigh the ingredients as you add them to the mixing pot.
For a roll of 55 towels, I multiplied the amounts by 56 (in order to account for the absorption by the cardboard roll in the center). Tip, if you measure the glycerin in a measuring cup, after you pour it out, place the cup in a microwave for a few seconds to heat the remaining glycerin, and the remainder will pour out easily).
We may not need this much OA in a towel. My preliminary testing with cardboard strips to replicate Maggi’s trials in Argentina indicated that it required 4 strips per box for adequate varroa reduction. That worked out to 40 g of OA per box, or 80 g per double-deep hive. A shop towel using the above formula holds only 12 g per hive. Its increased efficacy over the cardboard strips is apparently due to the chewing-removal action by the bees.
Tip: I noticed yesterday that it appeared to help to heat the water first, then add the OA. This appeared to allow me to break up the chunks better than when I add the OA to the hot glycerin first (I need to confirm this).
Update 14 Feb 2017
I’d had a number of people suggest rather complex manufacturing methods for spraying, vacuum suction, etc. methods for mass producing OA shop towels. I appreciate your suggestions, but I will leave mass production methods to manufacturers. What I’m focusing on are simple preparation methods for beekeepers. My most recent formulation is at the 12 Feb update.Some other recent questions:
Q: I don’t remember reading how long you leave this treatment in place.
A: In the first place, this application method is not yet approved for use in the U.S. You may be able to obtain an experimental use permit from your State department of pesticide regulation. That said, the application method is designed for an extended release over a period of roughly one month, by which point the bees should have removed most of the shop towel. Mite reduction is not immediate, and from my limited testing, appears to take about a month. Therefore, if legal in your state or country, apply it as soon as the mite infestation rate (as determined by alcohol wash) exceeds 2 mites/100 bees.
Q: Can it be used with supers in place?
A: According to Maggi’s testing, this treatment does not appear to increase OA residues in honey. I’m asking EPA to approve it for use with honey supers on.
Q: Does the ambient temperature matter?
A: I’ve tested at up to 100°F (at low humidity), and did not observe any adverse effects. I’ve also tested during our California winter (for adverse effects only–not efficacy), during sustained cold rain and some freezing temperatures. Again, I observed no adverse effects upon the bees or brood.
Q: When sourcing your Oxalic acid/Glycerin etc have you looked at different grades of OA? I see you have food grade gly. I have been sifting through OA msds sheets from what we have locally available and the chemical composition varies wildly from agricultural bore cleaner or wood bleach to technical grade OA with prices that vary even more. The lower end “bore cleaner” OA Dihydrate has Sulphate at 1000ppm and some metal elements included, Iron at 100ppm and Lead at 50ppm.
A: The EPA requires registered “Distributors” of OA for varroa treatment to certify the purity of the OA. Currently, Brushy Mountain is the only registered distributor. I’m working with another registrant to bring prepared towels to market at an inexpensive cost.
Update 12 Feb 2017
I checked several hives yesterday that have had a OA/gly towel in them for the past two months during rainy, cold spring buildup. There does not appear to be any adverse effect upon the colony.
Please remember that this formulation is not yet approved for use in the U.S. (I am in the process of obtaining an Experimental Use Permit for the State of California). It is against the law to apply OA to hives in an unapproved manner. I’m working with EPA and ARS to get this application method added to the label, but it’s a slow process.
Meanmwhile, I’ve been doing lots of kitchen chemistry to figure out how to avoid the need to press the excess OA/glycerin out of the shop towels in order to obtain a texture that the bees will remove. It appears the total glycerin should not exceed 13 mL per towel. This amount of glycerin will hold 12 g of OA, without the OA coming out of solution at hive temp.
I think that I’ve finally hit upon a formula that works. It is difficult to get the solution to wick by capillary action into the full length of a roll of shop towels. (For non U.S. readers, the Scott shop towels are a cellulose heavy-duty, highly absorbent towel http://www.kcprofessional.com/products/wipers/general/scott-shop-towels).
So here’s a step-by-step process that appears to make the ideal towel, although I need to confirm for efficacy in the hive.

Step 1. Use a sharp kitchen knife to cut the roll of towels exactly in half crosswise.

Step 2. Find a stainless steel saucepan that just fits the roll (stood on end). The optimal formula appears to be (per towel) 12 g of OA dihydrate, 13 mL of glycerine, and 5 mL of water. Multiplied by 55 towels + the cardboard roll = 56/2, for final amounts per half roll of towels of:
| OA | 336 g |
| glycerin | 364 mL |
| water | 140 mL |

Weigh out the 336 g of OA (I actually use a more accurate beam balance, but it’s not critical).

You can preheat the water and glycerin in the microwave, or on the stove. It doesn’t appear to be critical when you add the water, but if you add it first to the glycerin before adding the OA, it may help to prevent the formation of oxalic esters during the heating.

Heat to 140-160°F. Higher temps will cause degradation of the OA and accelerate ester formation (both of which we wish to avoid).

Add the OA crystals, and use a stainless steel spoon (it may discolor) to stir while you dissolve the OA. Wear nitrile gloves and safety glasses! Keep the burner on the mixture while stirring, but do not allow the temp to exceed 160F. With a modicum of caution, this is safe to do.

As soon as the mixture is completely clarified, remove it from the heat and place the pan on a plastic tray.

Preheat the roll of dry towels for 1 minute in the microwave–this preheating helps the solution to wick into the roll. Although you can insert a piece of 1-1/4″ PVC pipe into the roll, I find no benefit from doing so, since the cardboard cylinder will come loose anyway. Also no benefit to leaving the wrapper around the roll, since I get better absorption if the roll is allowed to expand.

Carefully place the roll into the hot solution. The pan should first be placed on a plastic tray (not as shown). The solution will quickly wick up part way. When it gets to about 1/3rd of the way up, then flip the roll.

Use kitchen tongs to flip the roll. The mixture will not immediately irritate your skin, and can be easily washed off with warm water. But it is sticky, and will get on all surfaces if you’re not careful. It is really important not to allow traces of the solution to get on surfaces, as it is slowly corrosive, and a hazard. You don’t want it ending up in your eyes! Keep warm water and a wet sponge on hand for wiping surfaces. A little bit of baking soda dissolved in water will immediately neutralize any acid.

The solution will wick up from the other end.

You may find it to be of benefit to flip the roll again. The roll will absorb every last drop of solution. Allow the roll to cool, and for the water to evaporate off (I’m not sure that this is necessary, as it would also occur later in the hive). You can then place the roll into a secure plastic container or ziplock bag for storage.
I found that when I held towels in the solution at 150F for a few days (with or without water), that the acid dissolved the towel. I don’t observe this happening at room temp, but have not had time to test to determine how long the treated towels can be stored before losing strength. The Aluen CAP strips claim to be good for an extended storage period. I will be performing tests to see whether the OA or towels degrade with extended storage. For now, probably best to make them up fresh.
One would then apply two half sheets of towel per hive for a treatment, which would apply 12 g of OA to the hive.
I’m not completely sold on the rolled towels, and have the folded version on order, which I will test when they arrive.
I’m buried in email from those wanting more info. Please only email me if you have something important to offer, or a serious question. If you are not comfortable with accurate weighing, measuring, and safe handling of acids, YOU SHOULD NOT ATTEMPT TO DO THIS!
I am encouraging one cooperating bee supply company to bring an inexpensive premade version to market.
Update 24 Jan 2017
There has been lots of response to my article on OA/gly in ABJ. I’m in communication with EPA to get this application method approved.
I’m in the middle of a trial of OA/gly shop towels on 6 hives during our wet, cold winter. As of today, after ~3 weeks, the colonies are not showing any adverse effects upon the bees.
I’m currently working on is the best ratio of OA to glycerin, and the best amount per towel. The problem is that the towels don’t want to evenly absorb the optimum amount of glycerin to leave them less “oily” so that the bees will chew at the towels. I’m trying to figure out how to get a full roll of towels to absorb the right amount of solution without the need for squeezing out the excess.
I tried 700g OA in 700ml of glycerin, heated to 160F, and the roll of towels preheated 1.5 minutes in the microwave. I poured the hot solution into a tall, narrow asparagus pot, and set one end of the towel roll into the pot. When the solution had soaked half way up, I flipped the roll over, and placed the roll into a warm oven. After 15 minutes, the solution has still not absorbed to the center of the roll. So I left it for another hour in the warm oven. This proved to be a mistake, since the acid degraded the lower part of the roll into a soft mess.
The 700ml of glycerin also appeared to be too much–leaving the towels too “oily” feeling.
I’m now going to experiment with adding isopropyl alcohol (boiling point 180F) to the solution, and decreasing the amount of glycerin.
